Scientists are often known for our tendency to let our professional lives intermingle with our home/family lives. One classic portrayal of this in fiction is Dr. Murray (the mother of the main character in Madeleine L’Engle’s A Wrinkle in Time), a microbiologist who is not yet done with her experiment when it’s time to start dinner . . . so she just cooks the stew on a bunsen burner in her home laboratory. (When I first read the book, many years ago, this struck me as a eminently reasonable approach, and it did not cross my mind for an instant that there might be any potential downside to this arrangement — e.g., worries about the stew contaminating her experiment, or vice versa.)
Dr. Murray’s kids were already school-aged, though. When your child is a baby, and you are spending time at home with it, there’s decent likelihood that — amidst all the feeding, soothing, diaper-changing, general mess-management, etc. — you will not have sufficient resources (time, energy, and/or access to antimatter or high-voltage power supplies) to make much progress on your real research projects. Thus, you might instead find yourself re-directing your “usual methods” to your current situation.
This might mean recording every time the baby is asleep, for an entire year, and then producing a plot of the data (broken down into 15-minute increments) that nicely illustrates how your newborn’s many shorter naps gradually coalesced into fewer, longer periods. Or it might mean meticulously charting breast-milk production, so as to test the frustrating and often contradictory claims that various experts have made to you about this process. Perhaps you merely ponder whether the not-quite-yet-fussing baby’s gradual motion/rotation on the floor can be well described by a random walk model. Or maybe you find yourself analyzing the color choices on your child’s wooden blocks that are decorated with the elements of the periodic table:

When you see this, what question(s) spring to mind?
I personally didn’t do all of those when I was home with my baby — just most of them. And although the baby in question is now 4.5 years old (but still loathe to let me focus my attention on complex physics questions in her presence), I recently found out that 2019 is the International Year of the Periodic Table of Chemical Elements, so I figured it was a good opportunity to dig out some of those old results.
The blocks in question were made the company Uncle Goose, which has all sorts of gorgeously decorated cubes of wood available for purchase. We received them as a gift from friends back when the recipient mainly just waved them around and tasted them (the latter being an activity that the manufacturer explains in its FAQs is technically safe but definitely not endorsed). This gave us, her parents, the opportunity to revel in making casual comments such as, “Looks like your daughter likes to gnaw on lithium — oh wait, now she’s moved on to testing the thallium.”
There were several things about the blocks that caught my eye as I was whiling away the brief lulls between child maintenance needs, mainly having to do with which elements were which colors:
- The noble gases were green . . . but then so was arsenic.
- Fluorine, chlorine, and bromine were red . . . but iodine was orange.
- Gallium and indium were different colors, as were arsenic and antimony.
In order to get to the bottom of how the colors were distributed, I took photos of the different faces and used the GIMP to combine and arrange the images appropriately. First for comparison, here is the version from Wikipedia’s “Periodic Table” page (pre-2016, though we’ll revisit that distinction a bit later):
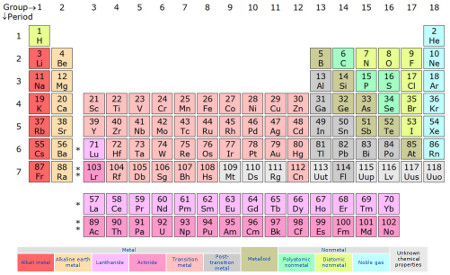 >(creator: Sandbh, license: CC BY-SA 4.0)
>(creator: Sandbh, license: CC BY-SA 4.0)And here’s the block version:

In hindsight, scanning them would have been a better choice for color uniformity. There are two sides not occupied by elements, since 20 cubical blocks have a total of 120 sides, and there are only 118 elements; they have the name of the block set and the Uncle Goose logo.
You can see that there are definitely suggestions of chemistry in the blocks’ color distribution.
- The diagonal boundary of the green area goes right through the diagonal region of the metalloids.
- There are no pink or purple blocks among the transition metals, while the alkali metals, alkaline earth metals, and post transition metals are almost exclusively pink and purple.
- Except for promethium, the lanthanides are either red or orange, and the actinides below them are either pink or purple, with the reds and pinks usually paired up and the oranges and purples usually paired up.
In many other ways though, I don’t see a good reason for color choices — e.g., what do the blue elements have in common? I was certain there had to be a more logical color arrangement . . .
Before we get to that, though, I will note there were a couple more things that caught my attention I was arranging and photographing the blocks:
- More often than not, it seemed like the lowest number on a block was green, while the highest was purple.
- No block had any two consecutive elements on it. (How likely is this to have happened by accident? Does it indicate that there was a deliberate effort to get a large spread of atomic numbers on each block?)
Given these initial impressions, the next step, of course, was to tabulate the data, make some graphs, and do some basic probability and statistics calculations.
After all, you know what they always say about parenting infants: “Spreadsheet while the baby sleeps.”
In case you’re curious about the results of those investigations, I’ve summarized them on a separate bonus page, so as to allow us to get back to what I think is the most fun question: If you need to color the elements with only six colors, and no more than 20 of each color, how can you do it so as to make the strongest connections to the chemistry of the periodic table?
This is the best I’ve been able to come up with:
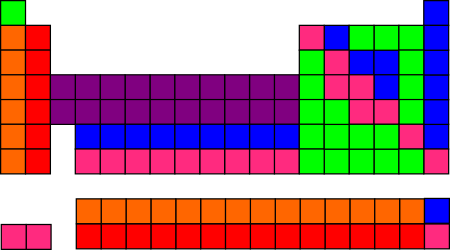
It’s not perfect, due to the constraints (6 colors, no more than 20 of each), but I think it looks pretty good! And it would look even better, aesthetically, if you draw the table with Lu and Lr under Y, as is sometimes done — e.g., in the Wikipedia table above.
Maybe I’ll send my proposal to Uncle Goose, in case they want to use it next time they do a block redesign. (The ones they sell now are not the same as my daughter’s, but they seem to have only changed which six colors they used, not how the colors are distributed.)
It’s also high time for an update. Although wooden blocks themselves are a fairly timeless toy for children, that’s not necessarily the case for things are printed on the side of them. Elements 113, 115, 117, and 118 received their official names and symbols more than two years ago. Thus, ununtrium (Uut), ununpentium (Uup), ununseptium (Uus), and ununoctium (Uuo) are now nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og), respectively. Given that the blocks are noticeably dated — and have been for a while — maybe this being the International Year of the Periodic Table of Chemical Elements would be the right time to come out with a new set.
Although putting elements on the sides of wooden blocks is never going to be the best way to learn about them, since it breaks up the arrangement of the periodic table (which conveys a great deal of information about the chemistry), maybe at least future scientist parents playing with their babies wouldn’t have to wonder why iodine is a different color than fluorine, chlorine, and bromine.
